Calls to reduce the heavy reliance on carbon-based fuels, such as coal, are loud and clear. The concern is that relying on carbon-based substances contributes to climate change and its dramatic effects – severe storms, hotter temperatures, rising sea levels, drought and more. But before burning out on coal completely, scientists at Ohio University experimented with it by examining if it could be morphed into graphite.
Why the coal-to-graphite conversion? Because scientists are aware that theoretically coal can be converted to graphite with enough pressure at high temperatures. Graphite, a material commonly used in pencils, is also used in batteries that fuel Tesla S and other electric vehicles known to directly reduce carbon emissions.
Additionally, graphite is a crystalline, stable form of carbon with a different atomic structure – it features stacked layers of graphene – not as messy as coal’s structure. So, the research team, led by David Drabold at Ohio University, simulated coal and graphite in computer software using the Bridges-2 advanced research computer at Pittsburgh Supercomputing Center.
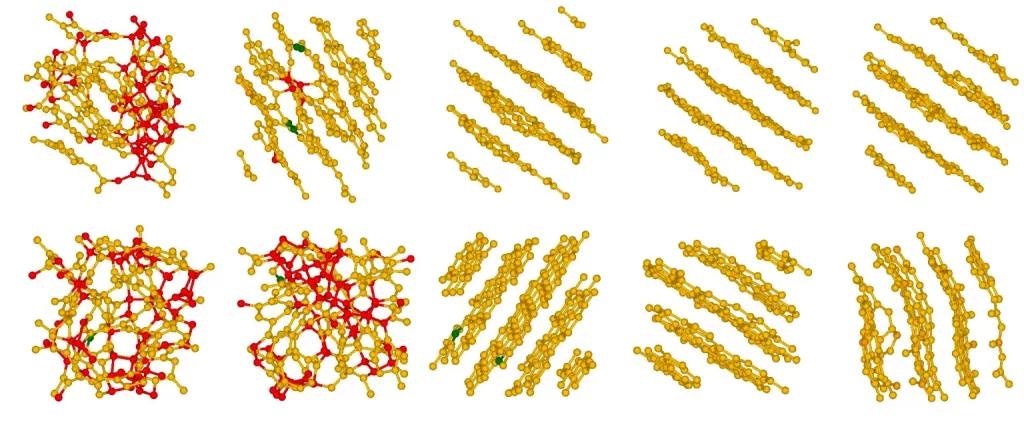
As part of their study, the researchers created a simplified version of “coal,” which they exposed to pressure and intense heat – about 5,000˚ Fahrenheit. According to the scientists, their results were more complicated and simpler than they expected. The findings, published in Physical Review Letters and basic solid state physics (pre-print), offer hopeful implications for converting coal to carbon-neutral materials for electronic and battery technologies.
To push out the amorphous-graphite paper we needed to do a lot of serious analysis. Compared to other systems which we have, Bridges is the fastest and most accurate. Our home systems … take about two weeks to simulate 160 atoms. With Bridges, we can run 400 atoms over six to seven days using density functional theory.
Chinonso Ugwumadu, physics doctoral student at Ohio University
You can read more about this story here (Published Jan. 5, 2023): Ohio University Simulations on PSC Supercomputer Transform Coal-Like Material to Amorphous Graphite and Nanotubes
If you have a project that could benefit from access to supercomputing resources like the ones used in this story, you can visit the allocations page to get started with ACCESS.
Project Details
Institution: PSC (Pittsburgh Supercomputing Center)
University: Ohio University
Funding Agency: U.S. Department of Energy (grant no. DE-FE0031981), NSF (grant no. ACI-1548562), XSEDE (allocation no. DMR-190008P).
Grant Numbers: DE-FE0031981, ACI-1548562, DMR-190008P
The science story featured here, allocated through August 31, 2022, was enabled through Extreme Science and Engineering Discovery Environment (XSEDE) and supported by National Science Foundation grant number #1548562. Projects allocated September 1, 2022 and beyond are enabled by the ACCESS program, which is supported by National Science Foundation grants #2138259, #2138286, #2138307, #2137603, and #2138296.


